Caproic Acid for Sustainable Polyol Esters with Low Viscosity and Low Volatility
In the world of lubricants, polyol esters (POEs) are renowned for their outstanding performance and exceptional versatility. These compounds are obtained by esterifying polyols with fatty acids. Their versatility lies in the fact that by thoughtfully choosing these building blocks, POEs with unique properties and performance can be crafted to meet the requirements of specific applications. However, such versatility is limited by the natural availability of these building blocks.
Linear fatty acids with chain lengths longer than six carbons (heptanoic- C7, caprylic – C8, capric -C10, etc.) are usually sourced from oleochemicals such as palm kernel, coconut oil or castor oil. As they are not abundantly present in vegetable oils, shorter fatty acids such as valeric acid (C5) are usually sourced from petrochemicals. Interestingly, both oleochemical and petrochemical sources contain little to no caproic acid (C6), hindering the possibility of producing larger volumes at a competitive price. As a result, POE products based on C6 are not widely available on the market, although they may have physical properties such as low viscosity, volatility and pour point that are interesting for certain applications within the lubricants industry.
Figure 1. Esterification of pentaerythritol and caproic acid (C6) to pentaerythritol tetrahexanoate.

ChainCraft uses a unique non-GMO fermentation process to upcycle food waste into valuable chemicals, focusing on the production of medium-chain fatty acids with varying chain lengths. Twelve thousand tons of C6 per year are expected from the full-scale factory, scheduled for commissioning in 2026, offering a stable supply of caproic acid to the industry. Thanks to this, C6 can become a novel component and building block, unlocking new possibilities for synthetic ester design.
To showcase the benefits and expected performance of a C6-based POE, three different pentaerythritol-based POEs were synthesized, each with different fatty acid chain lengths: C5, C6 and C7. The POEs were characterized and compared.
The three POE molecules were synthesized by esterification at 170 °C, using an ion exchange resin catalyst (Purolite CT269 and CT169) to reach quantitative reaction yields. After synthesis, the esters were distilled and purified.
The industry standard for the purity of ester base oils is typically greater than 98%. After a thorough purification, PE-C5 reached a purity of 98.2% and PE-C6 and PE-C7 reached 97.9%, as shown in Table 1. The remaining percentage mainly consists of incomplete pentaerythritol esters (tri-esters instead of the desired tetra-esters). It is also hypothesized that some esters of di- and tri-pentaerythritol are present. Acid values and water content met the requirements reported in the literature and the market standard.
Table 1. Purity, acid value and water content of the three synthesized POEs
| PE-C5 | PE-C6 | PE-C7 | |
|---|---|---|---|
| Water content (w/w%) | 0.01 | 0.01 | 0.02 |
| Acid value (mg KOH/g) | 0.05 | 0.01 | 0.37 |
| Purity (w/w%) | 98.2 | 97.9 | 97.9 |
All three batches of POEs had a light odor and were clear with a slight yellow color. The samples are shown in Figure 2, and CIE L*a*b* values are reported in Table 2.
Figure 2: The three POEs synthetized.

Due to the possibility of slight degradation of the catalyst during the synthesis, the final products were tested for residual sulfur; all samples were below 500 parts per million. This catalyst residue might have affected the base oil properties; however, adjustments in the synthesis process can easily avoid this issue.
Table 2. CIE L*a*b* values for the three samples
| PE-C5 | PE-C6 | PE-C7 | |
|---|---|---|---|
| L | 97.8 | 96.4 | 97.1 |
| a | 1.6 | 2.5 | 1.8 |
| b | 13.1 | 32.9 | 19.7 |
To assess the potential of PE-C6 as a base oil, its general physiochemical properties were assessed and compared to those of PE-C5 and PE-C7.
The kinematic viscosities of PE-C6 at 100 °C and 40 °C fall between those of PE-C5 and PE-C7 (see Figure 3). The same is observed for the viscosity index (VI) (see Figure 4). As expected, the viscosities and VIs increase with the length of the fatty acid chains.
Figure 3. Kinematic viscosities at 40° C and 100° C of PE-C5, PE-C6 and PE-C7
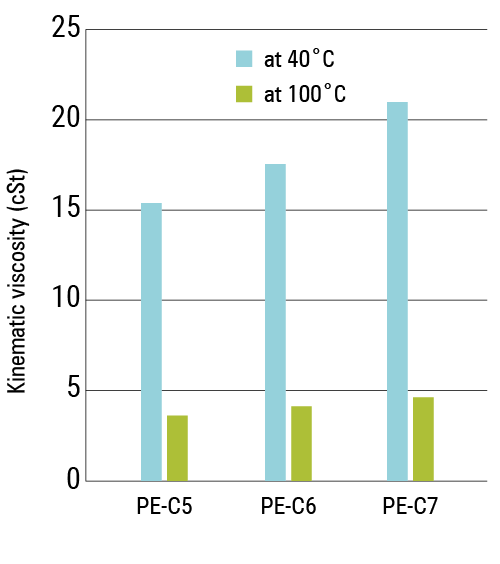
Base oils used in internal combustion engine (ICE) oils, electric vehicle (EV) fluids and aviation lubricants can benefit from lower viscosities, which can be achieved with small POEs. These applications also benefit from low volatility for safety and durability reasons. The volatility of all three molecules was found to be lower than 1.5% weight loss at 150 °C for 22 hours, as shown in Figure 5.
Figure 4. Viscosity index of PE-C5, PE-C6 and PE-C7

Compared to PE-C5, PE-C6 has a lower volatility, offering a better compromise between viscosity and volatility for many applications. Volatility is influenced by factors such as purity and molecular weight, with the expected trend being a decrease in volatility as the fatty acid chain length increases. However, the results showed an unexpected deviation: PE-C7 exhibited higher volatility than PE-C6, likely due to the lower purity of the PE-C7 batch, which had a slightly higher acid value and water content.
Figure 5. Volatility of PE-C5, PE-C6 and PE-C7
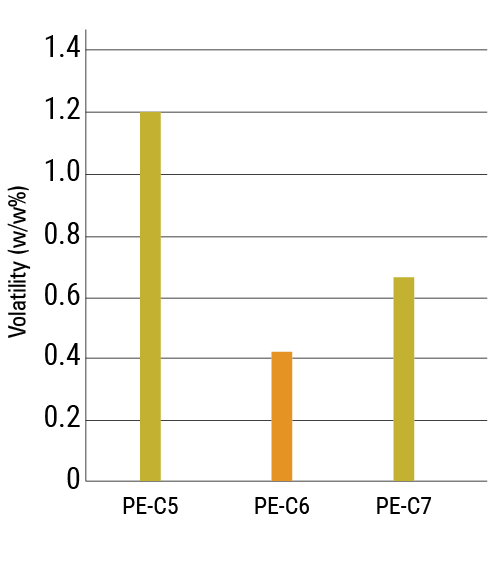
Flash point is another important parameter for applications where high temperatures can be reached and fire safety is important, such as transformer oils and hydraulic fluids. All three molecules achieved a flash point above 200°C, as shown in Table 3. While an increase in flash points with longer fatty acid chains was anticipated, PE-C5 showed a slightly higher flash point than both PE-C6 and PE-C7. It is hypothesized that this is due to the higher purity of PE-C5 compared to the other batches.
The performance of the base oil at low temperatures plays a crucial role in applications such as aviation and industrial lubricants. POEs generally perform well in such conditions, and PE-C6 reached a pour point of -36°C, as shown in Table 3. To further reduce the pour point, strategies such as using pour point depressants or incorporating shorter or branched fatty acids into the molecular structure could be considered. Unfortunately, the pour point of PE-C5 could not be assessed with certainty.
Table 3. Physicochemical properties of PE-C5, PE-C6 and PE-C7
| Property | Method | Unit | PE-C5 | PE-C6 | PE-C7 |
|---|---|---|---|---|---|
| Kinematic Viscosity @40 °C | ISO 3104 | cSt | 15.41 | 17.60 | 21.09 |
| Kinematic Viscosity @100 °C | ISO 3104 | cSt | 3.584 | 4.010 | 4.591 |
| Viscosity Index (VI) | ISO 2909 | 115 | 128 | 137 | |
| Pour Point | ISO 3016 | °C | -- | -36 | -21 |
| Flash Point | ISO 2719/A | °C | 213.0 | 202.5 | 204.5 |
| Volatility | ASTM D 972 | % (w/w) | 1.20 | 0.42 | 0.66 |
| Specific Gravity | EN ISO 12185 | kg/m³ | 1022.5 | 999.5 | 982.5 |
High-purity fatty acids are employed (>98%) for the synthesis of POEs at an industrial scale. Although fermentation is a novel way of deriving fatty acids for the lubricants industry, this production method does not negatively affect the quality of the product, and the highest purities can still be achieved.
The C6 that ChainCraft offers for industrial applications has a purity of over 98%. The remaining volume is comprised of other fatty acids with chain lengths ranging from C4 to C8 due to the nature of the fermentation process.
Lower your CO2 Footprint
Beside the unique characteristics of PE-C6, there is also a significant environmental benefit in using food waste-derived fatty acids for ester base oils.
As shown in Figure 6, a cradle-to-gate life cycle assessment (LCA) indicates that fatty acids produced from potato residues via ChainCraft’s fermentation technique have a carbon footprint three to six times lower compared to fatty acids derived from more traditional sources such as palm kernel oil, coconut oil and crude oil. Also, upcycling residual food streams into fatty acids does not take up arable land or compete with food sources.
Figure 6. Comparison of the global warming impact, excluding biogenic uptake, of fatty acids from different sources. More
detailed LCA results are available upon request.

Switching to starting materials with a lower carbon footprint to produce base oils allows companies to reduce their Scope 3 emissions. Furthermore, these fermentation-derived acids allow for POEs with a 100% bio-based carbon content, facilitating EU ecolabel “bio-based” and “bio-lubricant” claims.
Additionally, linear POEs excel for their biodegradability—which can reach up to 100% depending on the molecular structure—and have very low ecotoxicity. This makes them suitable for use in EALs (environmentally acceptable lubricants), which are employed in applications where the lubricants come into contact with the environment (total loss, partial loss and accidental loss lubricants). Examples include marine lubricants and lubricants used in outdoor machinery for agriculture and forestry. POE base oils can also be suitable for food-grade lubrication (NSF H1).
Registration Versus Innovation
Innovation can sometimes experience hurdles in terms of registrations. Fortunately, PE-C6 is included in existing POE REACH registrations. However, the technical innovation that C6 offers does not have to stop there; C6 is an innovative building block that can be utilized in the production of POEs not only based on pentaerythritol but also other polyols. Additionally, it can be used in the synthesis of complex esters and polymeric molecules to produce, for example, bio-based greases.
Unlocking the Middle Ground Between PE-C5 and PE-C7
Testing shows that the synthesized POE based on C6 has a viscosity that falls between PE-C5 and PE-C7. Also, the volatility of PE-C6 is lower compared to PE-C5. A low pour point combined with the balance between low viscosity and low volatility could allow the use of PE-C6 in low-temperature applications where the higher volatility of PE-C5 poses a safety risk.
Additionally, the expected environmental safety and biodegradability of PE-C6 can also offer advantages over existing materials in the market. Other properties of the PE-C6 may prove interesting to a wide variety of applications, such as dielectric properties for use in immersion cooling, chemical compatibility for use in refrigerant fluids and thermo-oxidative stability for use in the automotive industry.
Table 4. Composition of X-Craft® 6 with >98% C6 purity for industrial applications
These results only begin to show the unique benefits that can be achieved by using C6 in lubricant esters—and this is just the starting point. Ester chemistry is incredibly versatile, with a multitude of polyols available to synthesize new molecules with unique characteristics. C6 can also be combined with other fatty acids of higher chain lengths or molecules with different functional groups.
Upcycling food waste to an abundant supply of caproic acid opens up opportunities to produce sustainable synthetic esters for use in various lubricant applications. If you are interested in learning more about ChainCraft and its offerings, please visit www.chaincraft.com, or contact info@chaincraft.com.
Thomas Blundell is the product manager for ChainCraft and has a breadth of experience in the specialty and sustainable chemicals industry, working for companies such as Croda and Cargill Bioindustrial.
Maddalena Cesaro is an application scientist with a background in material science. She contributes to ChainCraft’s mission of promoting innovative and circular solutions by exploring new applications for its product line.
Erwin Zwolsman is a business developer at ChainCraft, leveraging a master’s in Biochemical Engineering to drive the commercialization of sustainably produced chemicals.
